Durable, Complete Remission
REZLIDHIA provided durable, complete remission, and improvement in transfusion independence
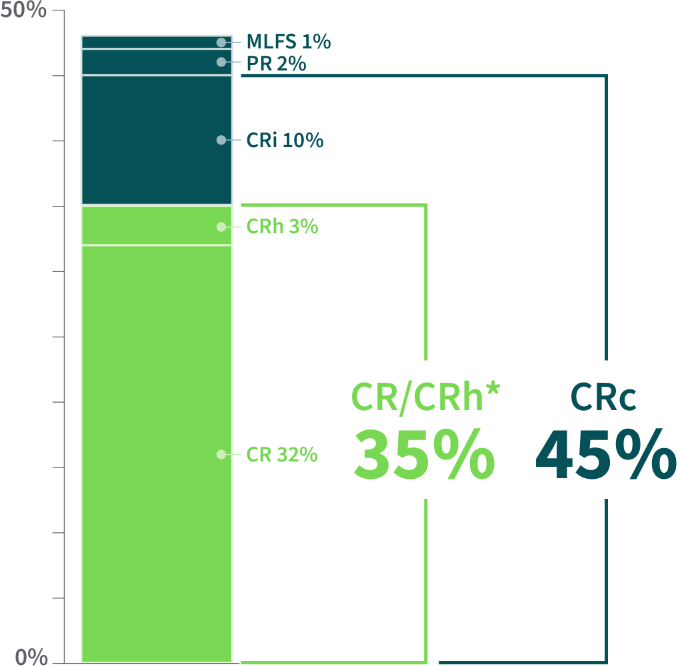
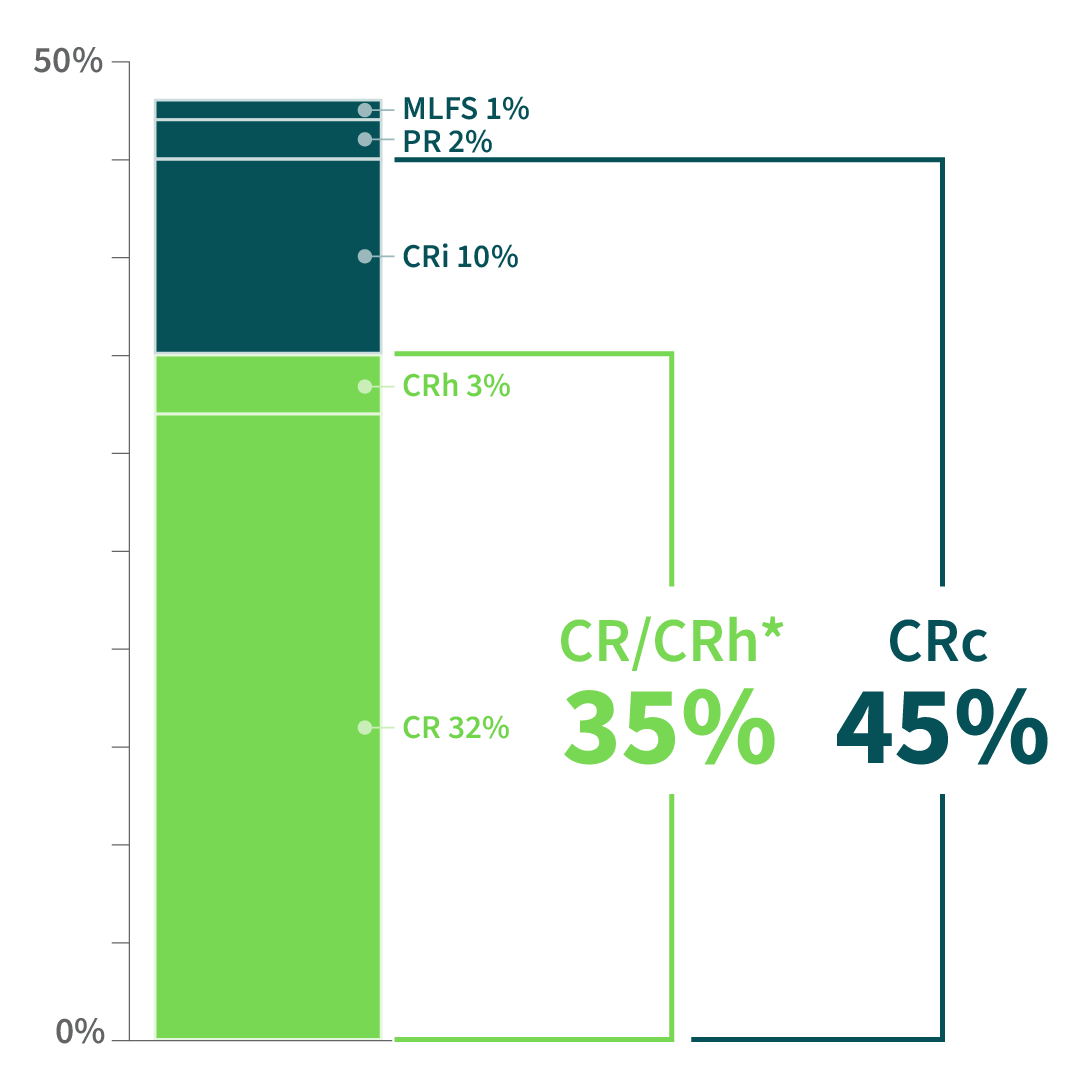
ALMOST HALF OF PATIENTS EXPERIENCED A COMPOSITE COMPLETE REMISSION
Clinical Response Rates From the REZLIDHIA Pivotal Trial (N=147)1,2
Additional Efficacy Outcomes1
- 11% (16 of 147) of patients became eligible for stem cell transplant after treatment
- Median time to CR or CRh: 1.9 months (range: 0.9-5.6 months)
The majority of responders achievedCOMPLETE REMISSION2
Primary endpoint.1
CR=complete remission; CRh=complete remission with partial hematologic recovery; CRi=complete remission with incomplete blood count recovery; CRc=composite complete remission (CR+CRh+CRi); MLFS=morphologic leukemia–free state; PR=partial remission (which required recovery of both neutrophil and platelet counts consistent with a CR).
OUTCOMES FOR PATIENTS WITH AML AFTER VENETOCLAX-BASED REGIMENS ARE OFTEN DISMAL
Response to REZLIDHIA in a subgroup of patients previously treated with venetoclax-based therapy was consistent with the pivotal trial.2,3-10
MEDIAN DURATION OF RESPONSE IN PATIENTS ACHIEVING CR/CRh WAS GREATER THAN 2 YEARS
Duration of CR/CRh Response2
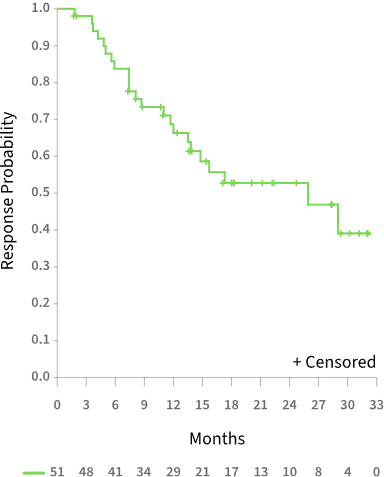
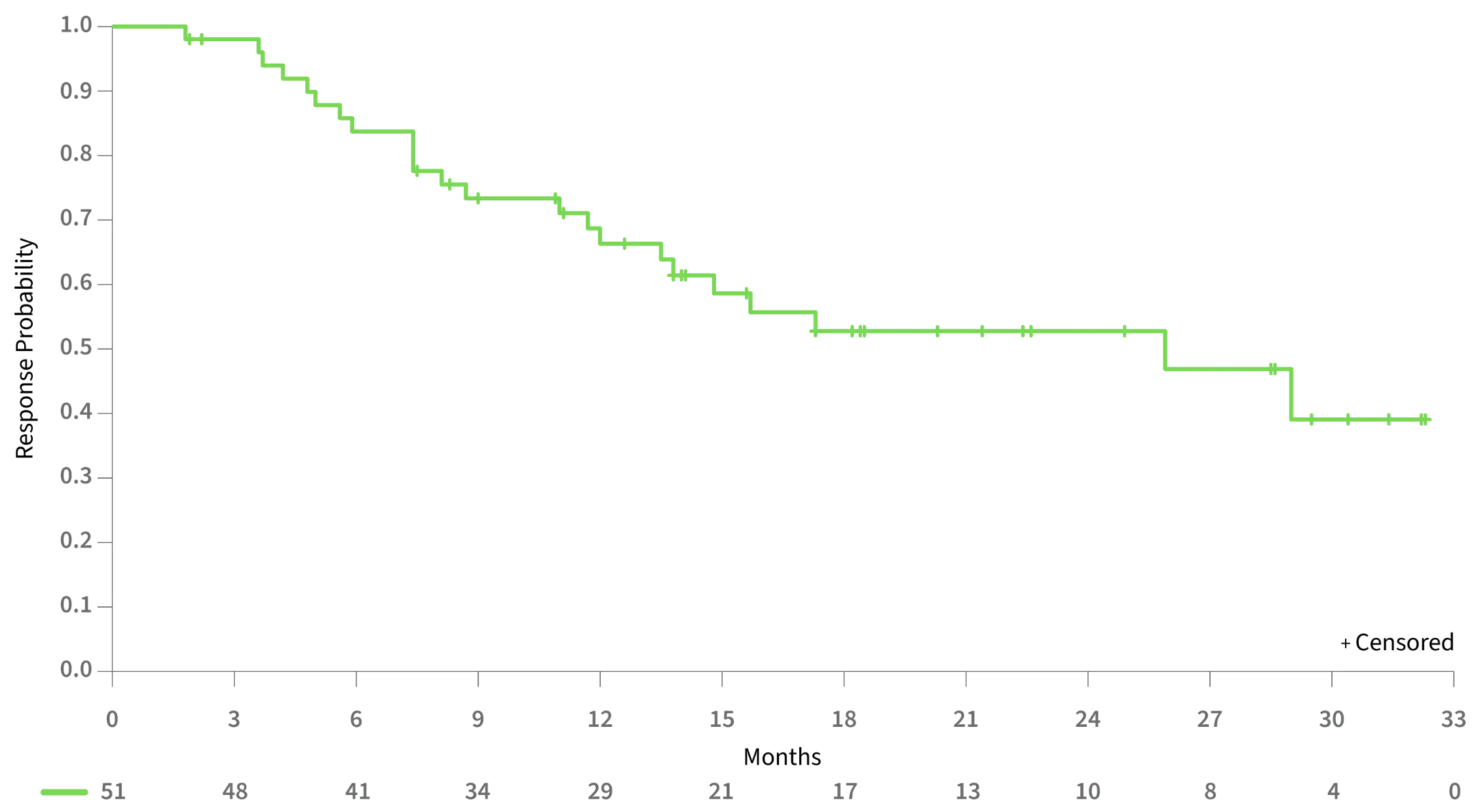
25.9 MONTHSMedian duration of CR/CRh1
28.1 MONTHSMedian duration of CR1
IMPROVEMENT IN TRANSFUSION INDEPENDENCE WAS SEEN ACROSS ALL PATIENT GROUPS
34% of transfusion-dependent patients became transfusion independent1,2
Patient Outcomes1,2
- Of the 86 patients who were transfusion dependent at baseline, a 56-day TI was achieved in 29 (34%), including patients in all response groups
- Of the 61 patients who were independent of both RBC and platelet transfusions at baseline, 39 (64%) remained transfusion independent
Other responders included patients achieving CRi, PR, or MLFS.
Percentage of Patients Achieving ≥56-Day Transfusion Independence (TI)2
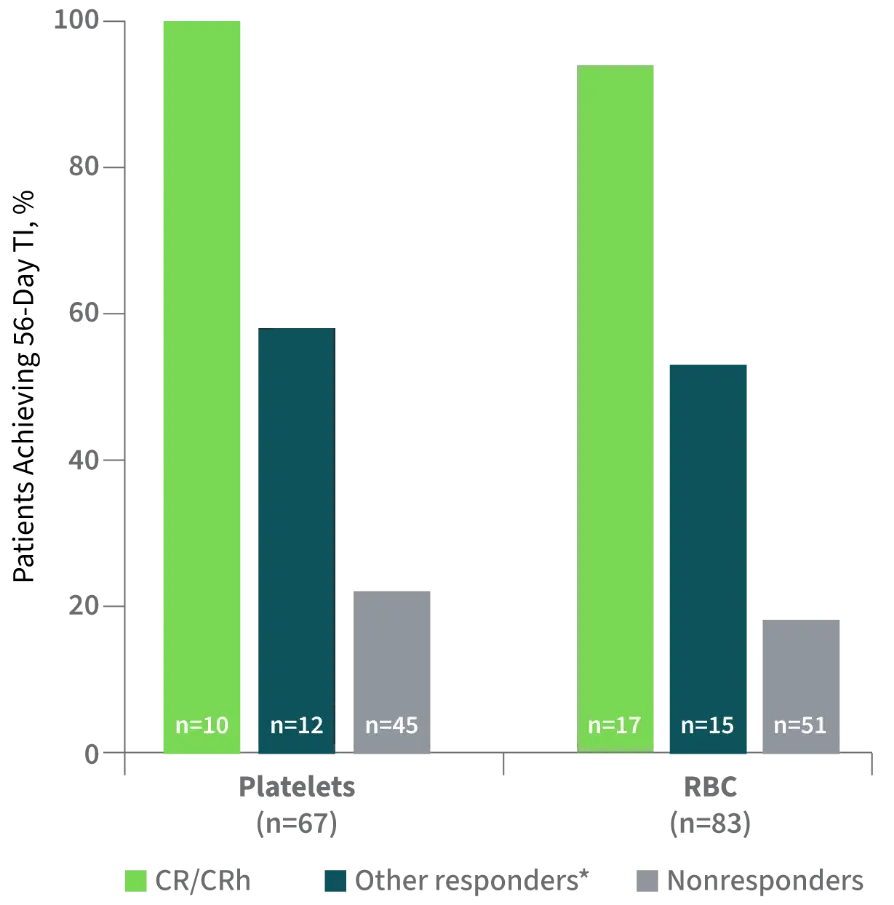
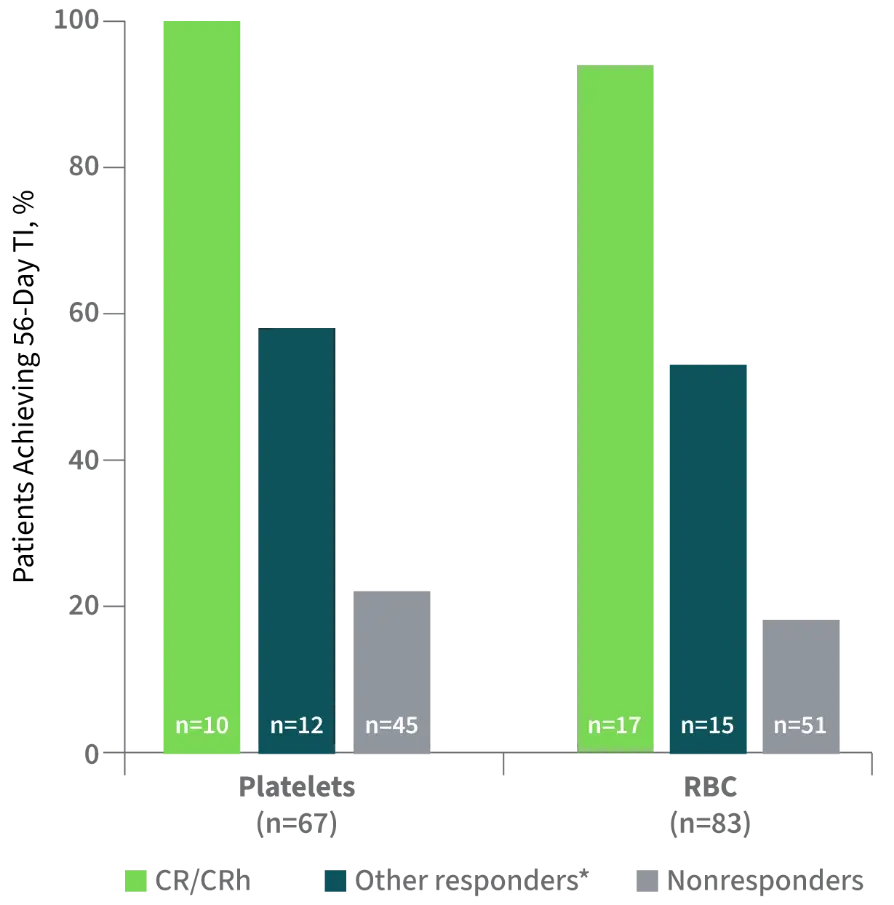
Other responders included patients achieving CRi, PR, or MLFS.
The observed efficacy is clinically meaningful and represents a therapeutic advance in this molecularly defined patient population with a poor prognosis and limited treatment options.
—de Botton et al. Blood Adv. 2023.2
CLINICAL TRIAL: STUDY 2102‑HEM‑101
REZLIDHIA was studied in an open-label, single-arm, multicenter trial in patients representative of those seen in clinical practice.1,2
| Patient Population (N=153)2 |
|---|
Adults (≥18 years old) Relapsed/refractory AML Confirmed IDH1 mutation No prior IDH1-inhibitor therapy ECOG PS 0-2 |
| Dosing1,2 |
REZLIDHIA* 150 mg Twice daily, orally Treated for ≥6 months, until disease |
| Clinical Endpoints2 |
Primary Endpoint Rate of CR/CRh† Secondary Endpoints
|
| Patient Population (N=153)2 | Dosing1,2 | Clinical Endpoints2 |
|---|---|---|
Adults (≥18 years old) Relapsed/refractory AML Confirmed IDH1 mutation No prior IDH1-inhibitor therapy ECOG PS 0-2 | REZLIDHIA* 150 mg Twice daily, orally Treated for ≥6 months, until disease | Primary Endpoint Rate of CR/CRh† Secondary Endpoints
|
REZLIDHIA was taken by mouth twice daily over continuous 28-day cycles, with doses at least 8 hours apart on an empty stomach.
Defined as bone marrow blasts <5% with absolute neutrophil count >500/microliter and platelet count >50,000/microliter.
DOR was calculated from the time of first response until death, relapse, or new anti-cancer therapy; patients without an event were censored at their last response assessment. Patients who discontinued therapy to proceed to hematopoietic stem cell transplantation (HSCT) were followed for DOR and OS, as HSCT was not an event.
Patients were classified as “transfusion dependent” if platelet and/or red blood cell (RBC) transfusion occurred within 56 days prior to baseline, and were transfusion independent if without platelet and/or RBC transfusions for at least 56 days during treatment.
Patients were enrolled between April 2018 and June 2020. Data cutoff=June 18, 2021.
ECOG PS=Eastern Cooperative Oncology Group Performance Status.
| Patient Demographic and Baseline Disease Characteristics | Efficacy-Evaluable Population (N=147) |
|---|
Demographics Age (Years), Median (Min, Max) 71 (32, 87) Age Categories, n (%) <65 years 37 (25) ≥65 years to <75 years 65 (44) ≥75 years 45 (31) Sex, n (%) Male 74 (50) Female 73 (50) Disease Characteristics ECOG PS, n (%) 0 45 (31) 1 76 (52) 2 23 (16) IDH1 Mutation, n (%)* R132C 85 (58) R132H 35 (24) R132G 12 (8) R132S 11 (7) R132L 4 (3) Type of AML, n (%) De novo AML 97 (66) Secondary AML 50 (34) Comutations, n (%)† NPM1 31 (21) FLT3 15 (10) TP53 9 (6) Cytogenetic Risk Status, n (%)‡ Favorable 6 (4) Intermediate 107 (73) Poor 25 (17) Unknown 9 (6) | Disease Characteristics (Cont’d) Prior AML Therapy Outcome, n (%) Refractory 46 (31) Relapsed 96 (65) Remission duration 67 (70) Remission duration 29 (30) Relapse Patients, n (%) 1 87 (59) 2 11 (8) ≥3 3 (2)
Transfusion Dependent at 86 (59) Prior Treatments Number of Prior Treatments Median (range) 2 (1-7) 1 regimen, n (%) 48 (33) 2 regimens, n (%) 45 (31) ≥3 regimens, n (%) 54 (37) Prior Treatments Received, n (%)‖ Cytarabine 105 (71) Idarubicin 64 (44) Daunorubicin 31 (21) Fludarabine 25 (17) Hypomethylating agent 58 (39) As a single agent 21 (14) Gemtuzumab-based 11 (7) Venetoclax-based 12 (8) With a hypomethylating 8 (5)
Prior Stem Cell Transplantation 17 (12) |
Using central IDH1 assay testing results.
Mutations other than IDH1.
Cytogenetic risk categorization was investigator reported by National Comprehensive Cancer Network® (NCCN®) or European LeukemiaNet (ELN) guidelines.
Transfusion dependent at baseline is defined as receiving a transfusion within 56 days prior to first dose of olutasidenib or noting transfusion dependence prior to coming on study.
ECOG PS=Eastern Cooperative Oncology Group Performance Status.

Olutasidenib (REZLIDHIA) is recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) as a targeted treatment option for relapsed/refractory AML with an IDH1 mutation.12
References:
- REZLIDHIA®. Package insert. Rigel Pharmaceuticals, Inc; 2022.
- de Botton S, Fenaux P, Yee K, et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. 2023;7(13):3117-3127. doi:10.1182/bloodadvances.2022009411
- Cortes J, Jonas BA, Schiller G, et al. Olutasidenib in post-venetoclax patients with mutant isocitrate dehydrogenase 1 (mIDH1) acute myeloid leukemia (AML). Leuk Lymphoma. Published online March 27, 2024. doi:10.1080/10428194.2024.2333451
- Bewersdorf JP, Shallis RM, Derkach A, et al. Efficacy of FLT3 and IDH1/2 inhibitors in patients with acute myeloid leukemia previously treated with venetoclax. Leuk Res. 2022;122:106942. doi:10.1016/j.leukres.2022.106942
- Abaza Y, Winer ES, Murthy GSG, et al. Clinical outcomes of hypomethylating agents plus venetoclax as frontline treatment in patients 75 years and older with acute myeloid leukemia: real-world data from eight US academic centers. Am J Hematol. Published online February 11, 2024. doi:10.1002/ajh.27231
- Thol F, Döhner H, Ganser A. How I treat refractory and relapsed acute myeloid leukemia. Blood. 2024;143(1):11-20. doi:10.1182/blood.2023022481
- Gangat N, Ilyas R, Johnson IM, et al. Outcome of patients with acute myeloid leukemia following failure of frontline venetoclax plus hypomethylating agent therapy. Haematologica. 2023;108(11):3170-3174. doi:10.3324/haematol.2022.282677
- Khanna V, Azenkot T, Liu SQ, et al. Outcomes with molecularly targeted agents as salvage therapy following frontline venetoclax + hypomethylating agent in adults with acute myeloid leukemia: a multicenter retrospective analysis. Leuk Res. 2023;131:107331. doi:10.1016/j.leukres.2023.107331
- Maiti A, Qiao W, Sasaki K, et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: a propensity score matched analysis stratified by risk of treatment-related mortality. Am J Hematol. 2021;96(3):282-291. doi:10.1002/ajh.26061
- Tenold ME, Moskoff BN, Benjamin DJ, et al. Outcomes of adults with relapsed/refractory acute myeloid leukemia treated with venetoclax plus hypomethylating agents at a comprehensive cancer center. Front Oncol. 2021;11:649209. doi:10.3389/fonc.2021.649209
- Data on file, Rigel Pharmaceuticals, Inc. January 2024.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed March 27, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
INDICATION AND IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
WARNING: DIFFERENTIATION SYNDROME Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain. If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution.
WARNINGS AND PRECAUTIONS
Differentiation Syndrome REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in 16% of patients, with grade 3 or 4 differentiation syndrome occurring in 8% of patients treated, and fatalities in 1% of patients. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal. Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption of REZLIDHIA. Differentiation syndrome occurred as early as 1 day and up to 18 months after REZLIDHIA initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, temporarily withhold REZLIDHIA and initiate systemic corticosteroids (e.g., dexamethasone 10 mg IV every 12 hours) for a minimum of 3 days and until resolution of signs and symptoms. If concomitant leukocytosis is observed, initiate treatment with hydroxyurea, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms. Differentiation syndrome may recur with premature discontinuation of corticosteroids and/or hydroxyurea treatment. Institute supportive measures and hemodynamic monitoring until improvement; withhold dose of REZLIDHIA and consider dose reduction based on recurrence.
Hepatotoxicity REZLIDHIA can cause hepatotoxicity, presenting as increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased blood alkaline phosphatase, and/or elevated bilirubin. Of 153 patients with relapsed or refractory AML who received REZLIDHIA, hepatotoxicity occurred in 23% of patients; 13% experienced grade 3 or 4 hepatotoxicity. One patient treated with REZLIDHIA in combination with azacitidine in the clinical trial, a combination for which REZLIDHIA is not indicated, died from complications of drug-induced liver injury. The median time to onset of hepatotoxicity in patients with relapsed or refractory AML treated with REZLIDHIA was 1.2 months (range: 1 day to 17.5 months) after REZLIDHIA initiation, and the median time to resolution was 12 days (range: 1 day to 17 months). The most common hepatotoxicities were elevations of ALT, AST, blood alkaline phosphatase, and blood bilirubin.
Monitor patients frequently for clinical symptoms of hepatic dysfunction such as fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. Obtain baseline liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months, once every other week for the third month, once in the fourth month, and once every other month for the duration of therapy. If hepatic dysfunction occurs, withhold, reduce, or permanently discontinue REZLIDHIA based on recurrence/severity.
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, were aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia , rash, lipase increased, mucositis, diarrhea and transaminitis.
DRUG INTERACTIONS
- Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers.
- Avoid concomitant use of REZLIDHIA with sensitive CYP3A substrates unless otherwise instructed in the substrates prescribing information. If concomitant use is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
LACTATION
Advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose.
GERIATRIC USE
No overall differences in effectiveness were observed between patients 65 years and older and younger patients. Compared to patients younger than 65 years of age, an increase in incidence of hepatotoxicity and hypertension was observed in patients ≥65 years of age.
HEPATIC IMPAIRMENT
In patients with mild or moderate hepatic impairment, closely monitor for increased probability of differentiation syndrome.
Please see Full Prescribing Information, including Boxed WARNING.
REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
WARNING: DIFFERENTIATION SYNDROME Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain. If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution.
WARNINGS AND PRECAUTIONS
Differentiation Syndrome REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in 16% of patients, with grade 3 or 4 differentiation syndrome occurring in 8% of patients treated, and fatalities in 1% of patients. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal. Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption of REZLIDHIA. Differentiation syndrome occurred as early as 1 day and up to 18 months after REZLIDHIA initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, temporarily withhold REZLIDHIA and initiate systemic corticosteroids (e.g., dexamethasone 10 mg IV every 12 hours) for a minimum of 3 days and until resolution of signs and symptoms. If concomitant leukocytosis is observed, initiate treatment with hydroxyurea, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms. Differentiation syndrome may recur with premature discontinuation of corticosteroids and/or hydroxyurea treatment. Institute supportive measures and hemodynamic monitoring until improvement; withhold dose of REZLIDHIA and consider dose reduction based on recurrence.
Hepatotoxicity REZLIDHIA can cause hepatotoxicity, presenting as increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased blood alkaline phosphatase, and/or elevated bilirubin. Of 153 patients with relapsed or refractory AML who received REZLIDHIA, hepatotoxicity occurred in 23% of patients; 13% experienced grade 3 or 4 hepatotoxicity. One patient treated with REZLIDHIA in combination with azacitidine in the clinical trial, a combination for which REZLIDHIA is not indicated, died from complications of drug-induced liver injury. The median time to onset of hepatotoxicity in patients with relapsed or refractory AML treated with REZLIDHIA was 1.2 months (range: 1 day to 17.5 months) after REZLIDHIA initiation, and the median time to resolution was 12 days (range: 1 day to 17 months). The most common hepatotoxicities were elevations of ALT, AST, blood alkaline phosphatase, and blood bilirubin.
Monitor patients frequently for clinical symptoms of hepatic dysfunction such as fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. Obtain baseline liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months, once every other week for the third month, once in the fourth month, and once every other month for the duration of therapy. If hepatic dysfunction occurs, withhold, reduce, or permanently discontinue REZLIDHIA based on recurrence/severity.
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, were aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia, rash, lipase increased, mucositis, diarrhea and transaminitis.
DRUG INTERACTIONS
- Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers.
- Avoid concomitant use of REZLIDHIA with sensitive CYP3A substrates unless otherwise instructed in the substrates prescribing information. If concomitant use is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
LACTATION
Advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose.
GERIATRIC USE
No overall differences in effectiveness were observed between patients 65 years and older and younger patients. Compared to patients younger than 65 years of age, an increase in incidence of hepatotoxicity and hypertension was observed in patients ≥65 years of age.
HEPATIC IMPAIRMENT
In patients with mild or moderate hepatic impairment, closely monitor for increased probability of differentiation syndrome.
Please see Full Prescribing Information, including Boxed WARNING.