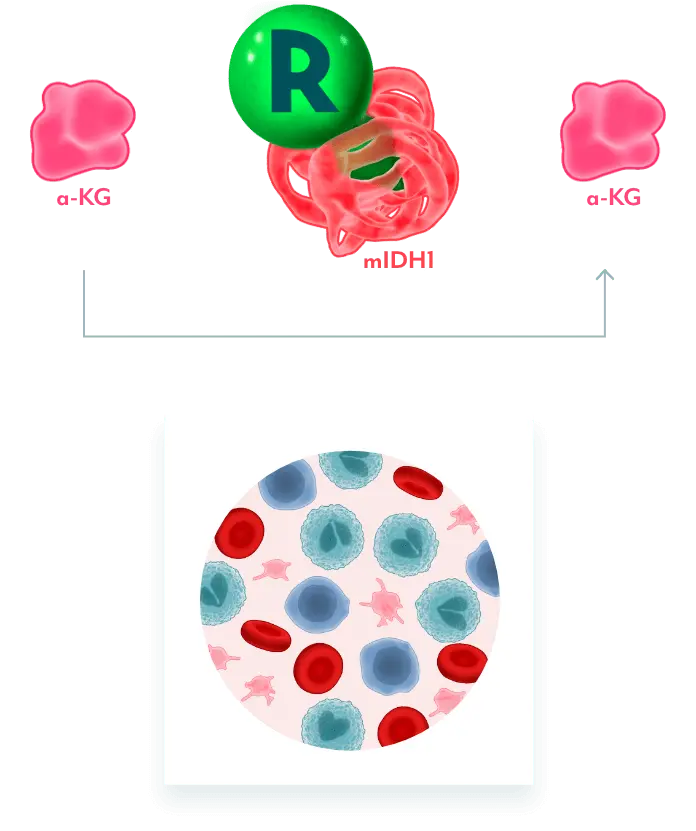
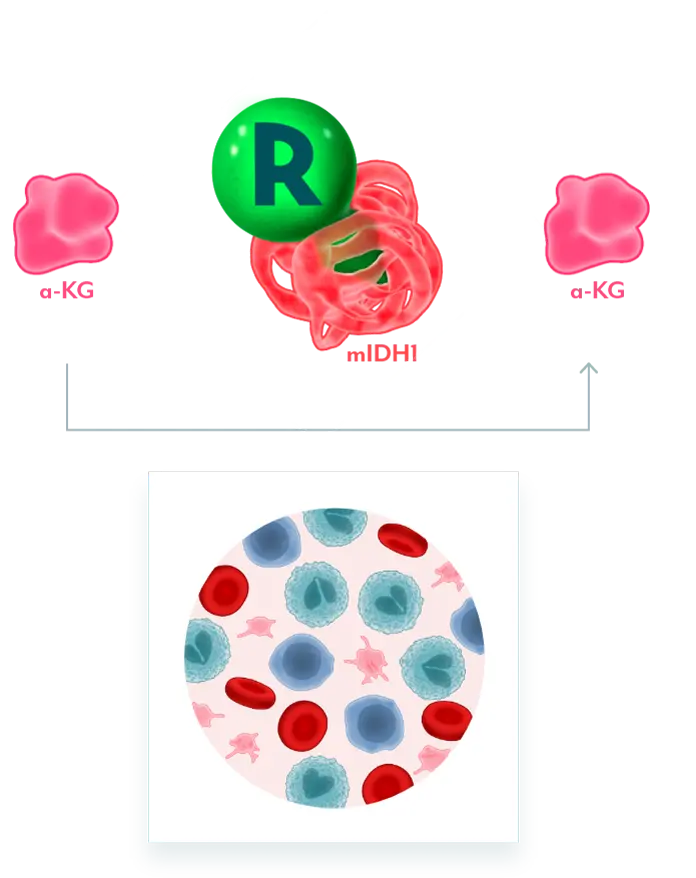
INHIBITS MUTANT IDH1 AND RESTORES MYELOBLAST DIFFERENTIATION
REZLIDHIA targets mIDH1 to allow for normal cellular differentiation

The NCCN Guidelines recommend testing for actionable mutations susceptible to targeted therapy at diagnosis and relapse in patients with AML.1
REZLIDHIA MECHANISM OF ACTION
Normal Cellular Function2
Normal IDH1 converts isocitrate to α-KG, which facilitates normal cellular differentiation.
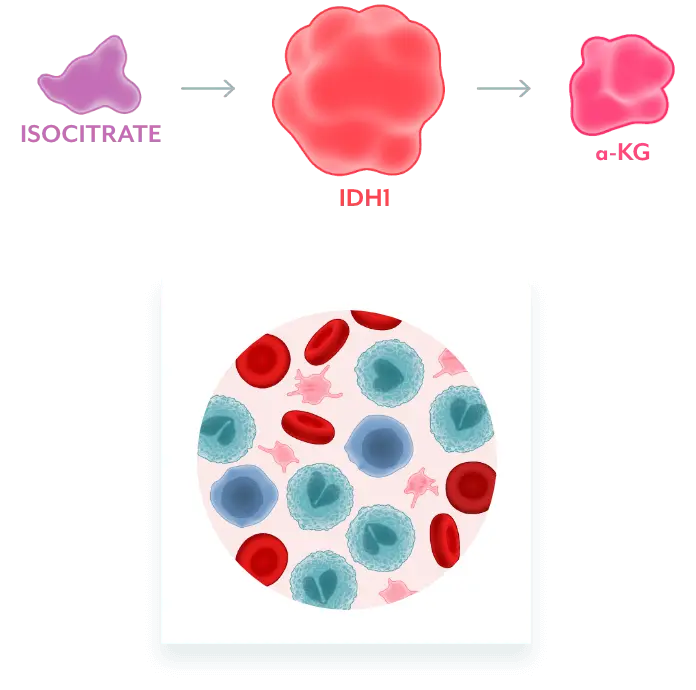
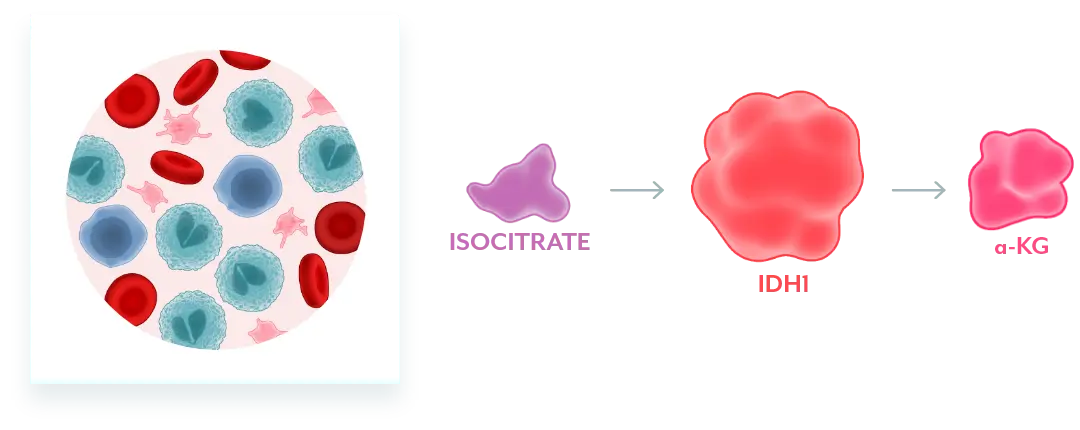
Mutant IDH1 Disrupts Normal Cellular Differentiation3-5
mIDH1 converts α-KG to 2-HG. Excess 2-HG, an oncometabolite, disrupts the function of enzymes necessary for myeloids to differentiate. This leads to disruption of normal cellular differentiation and results in an accumulation of myeloblasts.
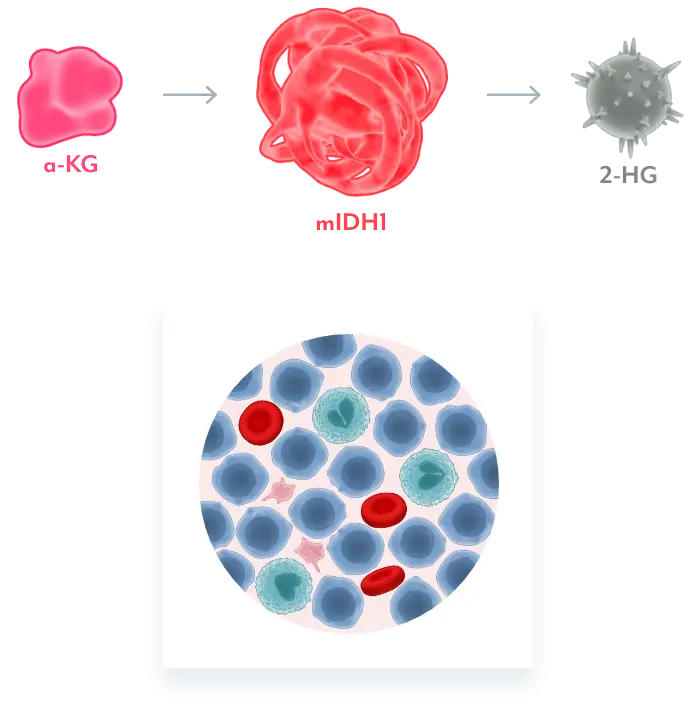
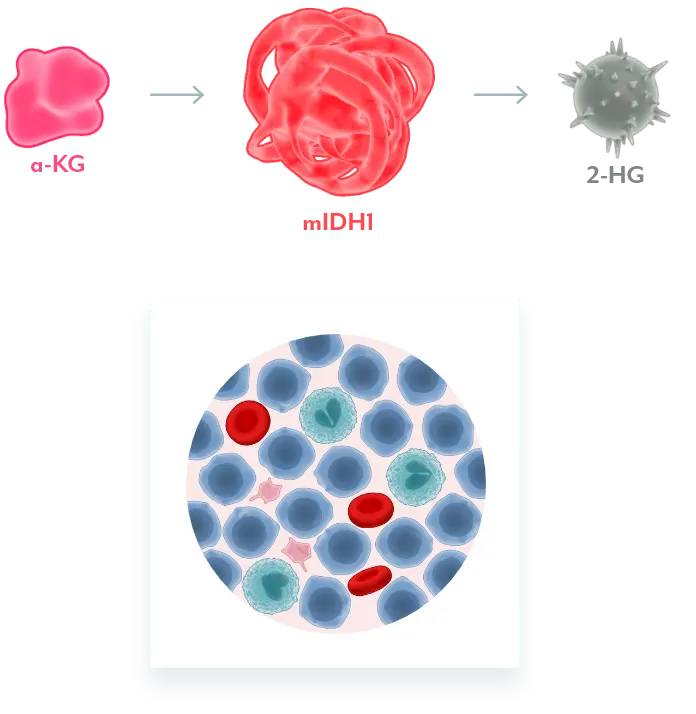
REZLIDHIA Restores Normal Cellular Differentiation3,4
REZLIDHIA is a potent, small-molecule inhibitor that binds to mIDH1, preventing the conversion of α-KG to 2-HG, allowing for normal cellular differentiation.

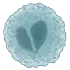
Myeloblast

Red blood cell
White blood cell
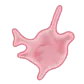
Platelet
References:
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed March 27, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932-941.
- REZLIDHIA® [package insert], South San Francisco, CA: Rigel Pharmaceuticals, Inc.
- de Botton S, Fenaux P, Yee K, et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. Published online February 1, 2023. doi:10.1182/bloodadvances.2022009411
- Liu S, Cadoux-Hudson T, Schofield CJ. Isocitrate dehydrogenase variants in cancer—Cellular consequences and therapeutic opportunities. Curr Opin Chem Biol. 2020;57:122-134.
INDICATION AND IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
WARNING: DIFFERENTIATION SYNDROME Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain. If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution.
WARNINGS AND PRECAUTIONS
Differentiation Syndrome REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in 16% of patients, with grade 3 or 4 differentiation syndrome occurring in 8% of patients treated, and fatalities in 1% of patients. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal. Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption of REZLIDHIA. Differentiation syndrome occurred as early as 1 day and up to 18 months after REZLIDHIA initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, temporarily withhold REZLIDHIA and initiate systemic corticosteroids (e.g., dexamethasone 10 mg IV every 12 hours) for a minimum of 3 days and until resolution of signs and symptoms. If concomitant leukocytosis is observed, initiate treatment with hydroxyurea, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms. Differentiation syndrome may recur with premature discontinuation of corticosteroids and/or hydroxyurea treatment. Institute supportive measures and hemodynamic monitoring until improvement; withhold dose of REZLIDHIA and consider dose reduction based on recurrence.
Hepatotoxicity REZLIDHIA can cause hepatotoxicity, presenting as increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased blood alkaline phosphatase, and/or elevated bilirubin. Of 153 patients with relapsed or refractory AML who received REZLIDHIA, hepatotoxicity occurred in 23% of patients; 13% experienced grade 3 or 4 hepatotoxicity. One patient treated with REZLIDHIA in combination with azacitidine in the clinical trial, a combination for which REZLIDHIA is not indicated, died from complications of drug-induced liver injury. The median time to onset of hepatotoxicity in patients with relapsed or refractory AML treated with REZLIDHIA was 1.2 months (range: 1 day to 17.5 months) after REZLIDHIA initiation, and the median time to resolution was 12 days (range: 1 day to 17 months). The most common hepatotoxicities were elevations of ALT, AST, blood alkaline phosphatase, and blood bilirubin.
Monitor patients frequently for clinical symptoms of hepatic dysfunction such as fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. Obtain baseline liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months, once every other week for the third month, once in the fourth month, and once every other month for the duration of therapy. If hepatic dysfunction occurs, withhold, reduce, or permanently discontinue REZLIDHIA based on recurrence/severity.
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, were aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia , rash, lipase increased, mucositis, diarrhea and transaminitis.
DRUG INTERACTIONS
- Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers.
- Avoid concomitant use of REZLIDHIA with sensitive CYP3A substrates unless otherwise instructed in the substrates prescribing information. If concomitant use is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
LACTATION
Advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose.
GERIATRIC USE
No overall differences in effectiveness were observed between patients 65 years and older and younger patients. Compared to patients younger than 65 years of age, an increase in incidence of hepatotoxicity and hypertension was observed in patients ≥65 years of age.
HEPATIC IMPAIRMENT
In patients with mild or moderate hepatic impairment, closely monitor for increased probability of differentiation syndrome.
Please see Full Prescribing Information, including Boxed WARNING.
REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
WARNING: DIFFERENTIATION SYNDROME Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain. If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution.
WARNINGS AND PRECAUTIONS
Differentiation Syndrome REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in 16% of patients, with grade 3 or 4 differentiation syndrome occurring in 8% of patients treated, and fatalities in 1% of patients. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal. Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption of REZLIDHIA. Differentiation syndrome occurred as early as 1 day and up to 18 months after REZLIDHIA initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, temporarily withhold REZLIDHIA and initiate systemic corticosteroids (e.g., dexamethasone 10 mg IV every 12 hours) for a minimum of 3 days and until resolution of signs and symptoms. If concomitant leukocytosis is observed, initiate treatment with hydroxyurea, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms. Differentiation syndrome may recur with premature discontinuation of corticosteroids and/or hydroxyurea treatment. Institute supportive measures and hemodynamic monitoring until improvement; withhold dose of REZLIDHIA and consider dose reduction based on recurrence.
Hepatotoxicity REZLIDHIA can cause hepatotoxicity, presenting as increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased blood alkaline phosphatase, and/or elevated bilirubin. Of 153 patients with relapsed or refractory AML who received REZLIDHIA, hepatotoxicity occurred in 23% of patients; 13% experienced grade 3 or 4 hepatotoxicity. One patient treated with REZLIDHIA in combination with azacitidine in the clinical trial, a combination for which REZLIDHIA is not indicated, died from complications of drug-induced liver injury. The median time to onset of hepatotoxicity in patients with relapsed or refractory AML treated with REZLIDHIA was 1.2 months (range: 1 day to 17.5 months) after REZLIDHIA initiation, and the median time to resolution was 12 days (range: 1 day to 17 months). The most common hepatotoxicities were elevations of ALT, AST, blood alkaline phosphatase, and blood bilirubin.
Monitor patients frequently for clinical symptoms of hepatic dysfunction such as fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. Obtain baseline liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months, once every other week for the third month, once in the fourth month, and once every other month for the duration of therapy. If hepatic dysfunction occurs, withhold, reduce, or permanently discontinue REZLIDHIA based on recurrence/severity.
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, were aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia, rash, lipase increased, mucositis, diarrhea and transaminitis.
DRUG INTERACTIONS
- Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers.
- Avoid concomitant use of REZLIDHIA with sensitive CYP3A substrates unless otherwise instructed in the substrates prescribing information. If concomitant use is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
LACTATION
Advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose.
GERIATRIC USE
No overall differences in effectiveness were observed between patients 65 years and older and younger patients. Compared to patients younger than 65 years of age, an increase in incidence of hepatotoxicity and hypertension was observed in patients ≥65 years of age.
HEPATIC IMPAIRMENT
In patients with mild or moderate hepatic impairment, closely monitor for increased probability of differentiation syndrome.
Please see Full Prescribing Information, including Boxed WARNING.